GMP制药生产,合规且高效
选择先进的传感器技术,保证产品质量,确保生产合规,提高生产效率
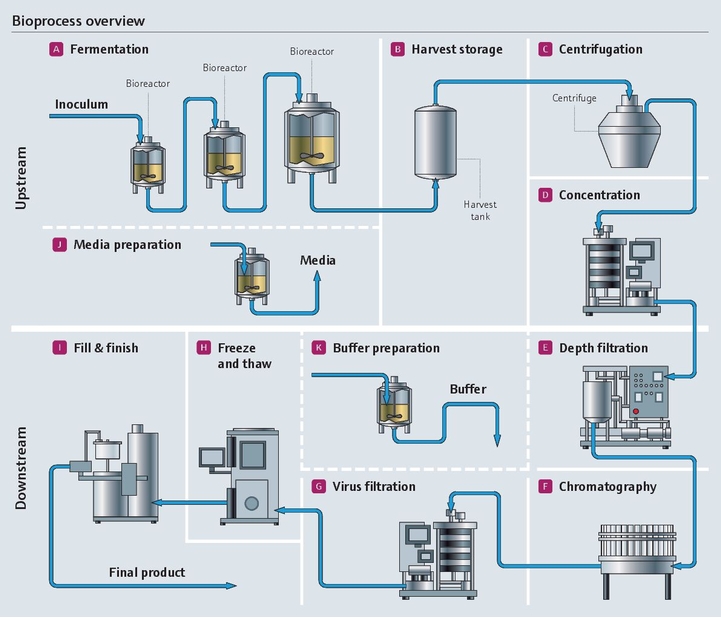
从小规模工艺研发到GMP制药生产的转换是极具挑战的。新仪表和新技术对应更高的自动化水平和控制水平,复杂性也相对有所提高。需要考虑GMP合规、行业标准和指南,才能确保产品质量达标。我们为制药行业的用户提供系列专用产品组合,能够实时监测和控制质量参数和工艺参数,并有效提高生产效率。
 ©Endress+Hauser
©Endress+Hauser
 ©Endress+Hauser
©Endress+Hauser
 ©Endress+Hauser
©Endress+Hauser
 ©Endress+Hauser
©Endress+Hauser
 ©Endress+Hauser
©Endress+Hauser
 ©Endress+Hauser
©Endress+Hauser
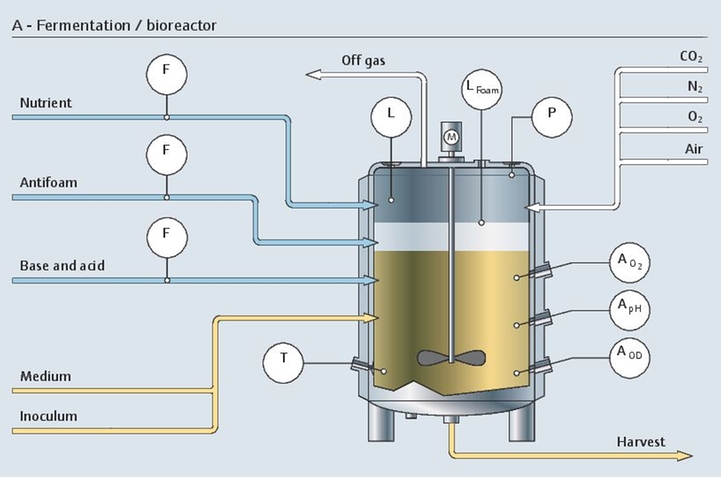
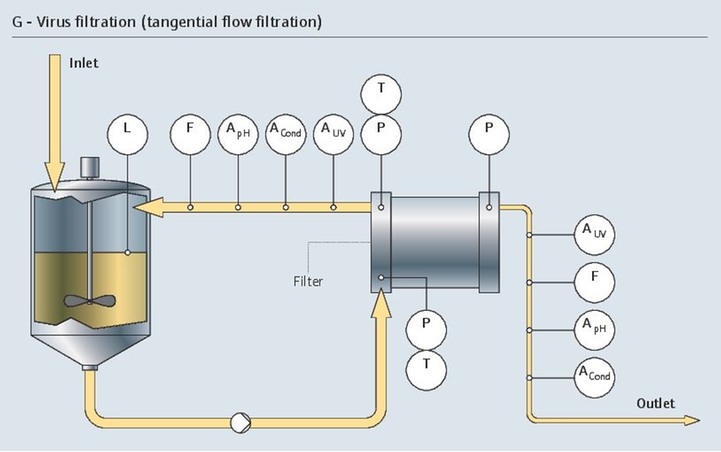
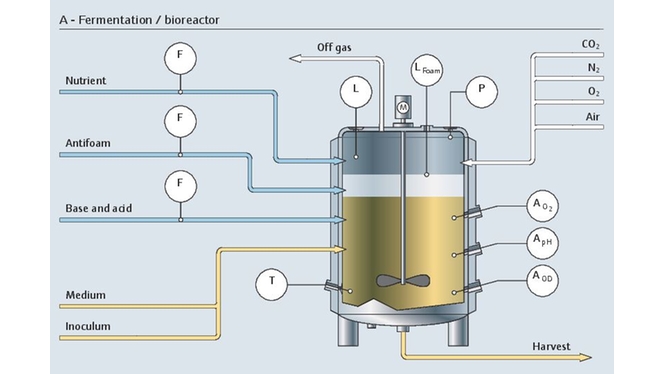
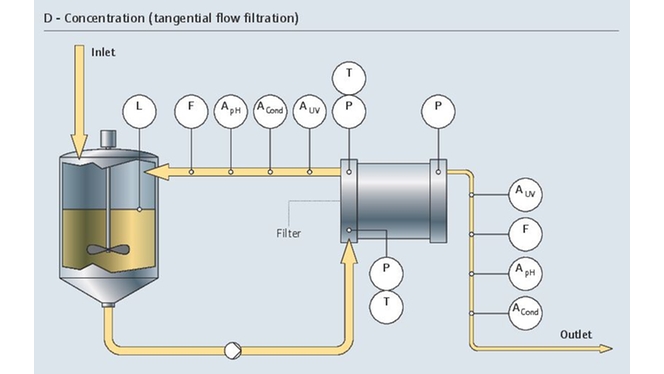
发酵涉及活细胞,是一个非常复杂、敏感、高成本的生物过程。整个生物制药过程在严格的无菌条件下进行。为了满足上述要求,稳定一致的测量结果至关重要。
我们的行业专业知识
我们的专用仪表组合具有高长期稳定性和高测量精度,完全能够控制生物反应器的状况:
 ©Endress+Hauser
©Endress+Hauser
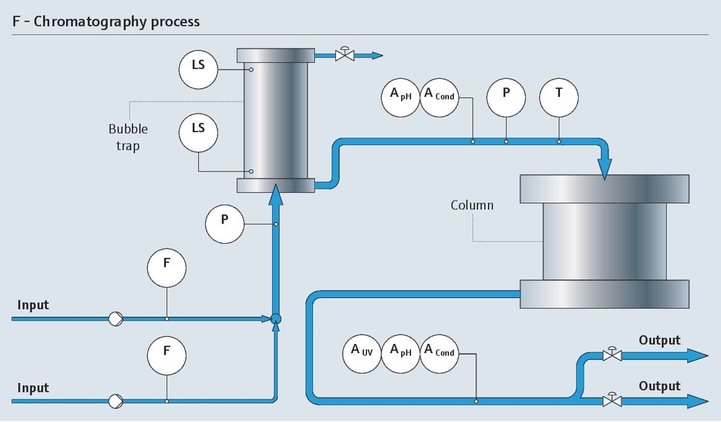
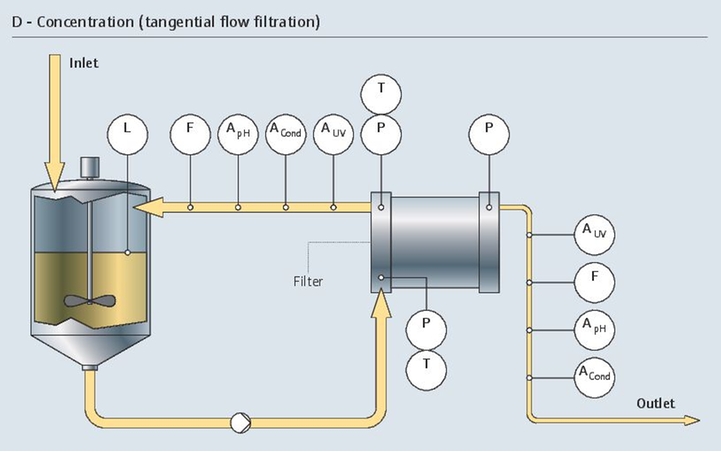
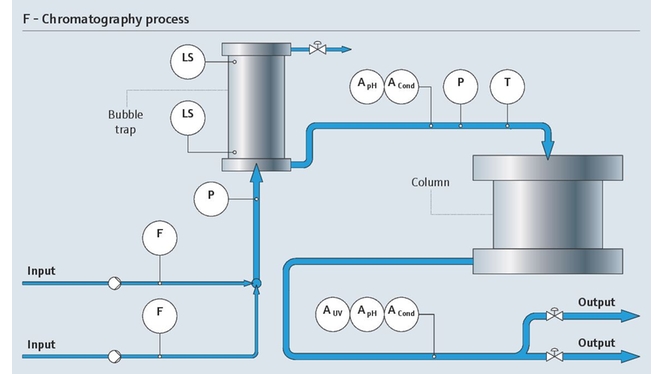
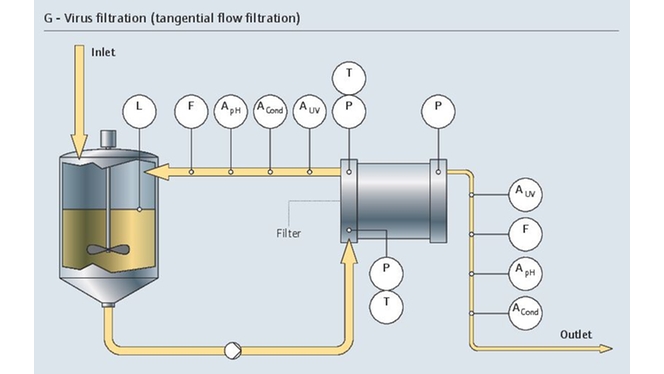
在层析工艺中,高精度电导率和pH测量至关重要。而且,检测出从发酵液的复杂混合液中分离出的目标蛋白质是一项难度很大的复杂挑战。
我们的行业专业知识
我们的监测解决方案通过维持所需层析条件,支持分离过程。我们的紫外光吸光度传感器能够检测出目标蛋白质:
- 在线测量pH和电导率
- 优化流量控制
- 防止工艺过压和出现超高温
 ©Endress+Hauser
©Endress+Hauser
pH值是生物反应器的关键控制参数。每类细胞都有最合适自己生长和蛋白质表达的pH值范围。从实验室到大规模生产都需要一致且可靠的数据。
我们的行业专业知识
Endress+Hauser的产品组合能够在发酵过程中稳定在线测量pH值,实现高可靠性,并支持实验室中的创新校准原理:
- 采用前沿的Memosens数字式传感器技术
- 从实验室到大规模生产过程,使用的传感器和仪表都采用相同的数字技术
优势
我们的员工Klaus Köhler先生向您介绍如何简化生物制药生产项目。他们不仅提供全系列仪表,而且拥有丰富的行业知识,按需为您提供理想的解决方案。
“我为Endress+Hauser的生物制药行业工作,十分荣幸能为您的项目提供专业支持。”
Klaus Köhler, 全球行业经理, Endress+Hauser德国
要点
我们帮助您
Endress+Hauser为制药行业的用户提供完整的测量仪表组合,方便灵活定制解决方案。我们的仪表和传感器能够精准监测关键工艺过程,确保生产合规,保证批次质量。
- 生物反应器和泡沫的液位测量和控制
- 层析设备中的蛋白质分离
- 实验室和生产过程中的分析测量
- 方便校准的现场型仪表
- 完全根据ASME BPE标准设计的系列仪表
除了所有相关测量和系统技术,我们还提供适当的软件,用于各个分支行业,确保您的操作过程满负荷运行。
光学分析系统,用于固体、液体、浆料、颗粒及气体的成分与浓度监测——适用于实验室、过程控制及排放监测领域。
对 水、天然气、蒸汽、矿物油、化学品进行测量是某些特定行业的日常工作,选择合适的流量计,满足您的过程需要。
对水、饮料、乳制品、化学品和药品进行分析是某些特定行业的日常工作。从Endress+Hauser 全面的产品系列中选择合适的仪表,满足您的过程需要。
专业知识加速化妆品生产过程
高效稳定的化妆品生产需要一个全方位服务提供商,他能够帮助您提高生产效率,缩短产品上市时间。

制药行业项目管理
嵌入式工程师帮助实现标准化,优化项目交付,降低运营成本。

我们非常重视您的隐私权
我们将Cookie用于提高网页浏览体验、收集统计数据来优化网站功能,以及投放定制化广告或内容。
选择“全部接受”,即表示您同意我们使用Cookie。
更多细节请查阅我们的 Cookie政策 。